创新背景
塑料废物在海洋、土壤甚至人体内的积累是现代的主要污染问题之一,到目前为止,已经处理了超过50亿吨。尽管人们在回收塑料产品方面做出了重大努力,但如何真正利用这种混杂的材料仍然是一个具有挑战性的问题。
一个关键问题是,塑料有很多不同的种类,将它们分解成可以以某种方式重复使用的形式的化学过程往往对每种塑料有特定的要求。从苏打水瓶到洗洁精罐再到塑料玩具,对这些大杂烩垃圾进行大规模分类是不切实际的。如今,通过回收项目收集的大部分塑料材料最终都被扔进了垃圾填埋场。
创新过程
根据麻省理工学院和其他地方的新研究发现有一个更好的方法。人们发现,一种使用基于钴的催化剂的化学过程可以非常有效地将各种塑料(如聚乙烯(PET)和聚丙烯(PP)这两种生产最广泛的塑料形式)分解为单一产品——丙烷。然后,丙烷可以用作炉灶、加热器和汽车的燃料,或作为生产多种产品的原料——包括新塑料,因此可能至少提供一个部分闭环回收系统。
回收塑料一直是一个棘手的问题,因为塑料中的长链分子是由碳键连接在一起的,碳键“非常稳定,很难分裂”。现有的打破这些键的技术往往会产生不同分子的随机混合,然后需要复杂的提炼方法才能分离出可用的特定化合物,但是没有办法控制在碳链的哪个位置打破分子。
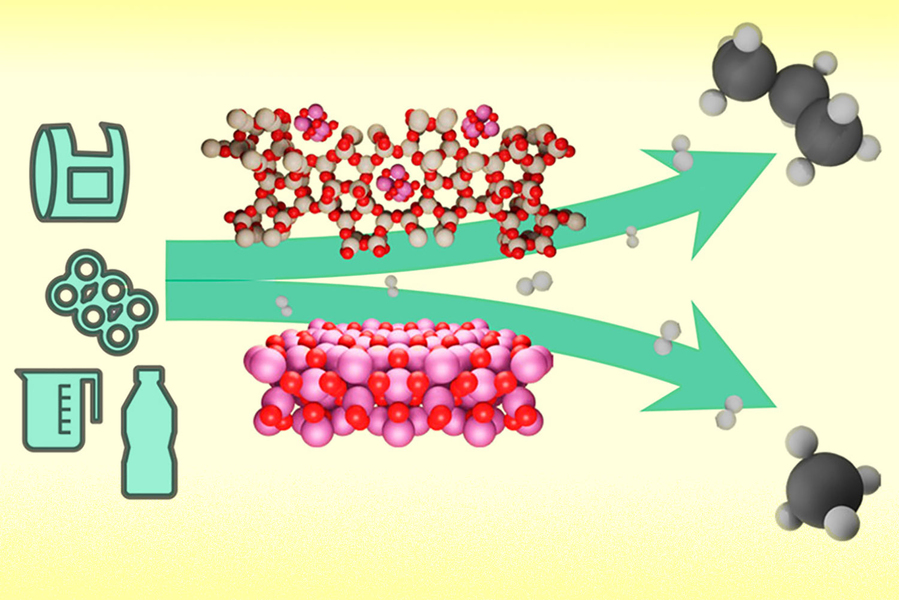
但研究人员发现,一种由含有钴纳米颗粒的微孔材料沸石制成的催化剂可以选择性地分解各种塑料聚合物分子,并将其中80%以上转化为丙烷。
虽然沸石充斥着细小的毛孔不到一纳米宽(对应于聚合物链的宽度),一个合乎逻辑的假设是,沸石和聚合物之间几乎没有相互作用。但情况恰恰相反:不仅聚合物链进入孔隙,而且钴和沸石中酸位点之间的协同作用可以在同一点上破坏链。事实证明,这个裂解位点对应于切除一个丙烷分子而不产生不需要的甲烷,让其余的较长的碳氢化合物一次又一次地准备经历这个过程。

研究人员表示,丙烷这种化合物可以减轻下游分离的负担。这一过程所需的材料沸石和钴都是廉价易得的。加拿大、古巴等地正在开发一些新产品。该过程所需的另一种材料是氢气,目前氢气主要来自化石燃料,但也可以很容易地通过其他方式获得,包括利用太阳能或风能等无碳电力对水进行电解。
研究人员在一个混合回收塑料的真实例子上测试了他们的系统,产生了有希望的结果。但是需要更多的测试更多种类的混合废物流来确定不同污染物的污染发生多少材料,如油墨、胶水、和标签附着在塑料容器或其他无塑性的材料混在一起浪费——以及它如何影响过程的长期稳定。
创新关键点
研究人员发现,一种由含有钴纳米颗粒的微孔材料沸石制成的催化剂可以选择性地分解各种塑料聚合物分子,并将其中80%以上转化为丙烷。
创新价值
这项新技术可以处理多种混杂的塑料垃圾,或许可以适应当今的塑料和混合废水处理系统。
New chemical processes use cobalt-based catalysts to achieve more efficient plastic recycling
There's a better way, according to new research from MIT and elsewhere. A chemical process using a cobalt-based catalyst has been found to be very efficient in breaking down various plastics such as polyethylene (PET) and polypropylene (PP), two of the most widely produced forms of plastic, into a single product, propane. Propane could then be used as fuel for stoves, heaters and cars, or as a feedstock for the production of a variety of products - including new plastics, and thus potentially provide at least a partial closed-loop recycling system.
Recycling plastic has always been a tricky issue because the long chains of molecules in plastic are held together by carbon bonds, which are "very stable and difficult to split." Existing techniques for breaking these bonds tend to produce a random mix of different molecules, which then require complex refining methods to isolate the specific compounds that are available, but there is no way to control where in the carbon chain the molecules are broken.
But researchers have found that a catalyst made of zeolite, a microporous material containing cobalt nanoparticles, can selectively break down various plastic polymer molecules and turn more than 80 percent of them into propane.
Although zeolites are filled with tiny pores less than a nanometer wide (corresponding to the width of the polymer chain), a logical hypothesis is that there is little interaction between zeolites and polymers. But the opposite is true: not only does the polymer chain enter the pore, but the synergistic interaction between the acid sites in cobalt and zeolite can break the chain at the same point. This cleavage site, it turns out, corresponds to excising a propane molecule without producing unwanted methane, leaving the rest of the longer hydrocarbons ready to go through the process again and again.
The researchers say propane is a compound that could ease the burden of downstream separation. Both zeolite and cobalt are cheap and readily available. Some new products are being developed in places like Canada and Cuba. The other material needed for the process is hydrogen, which currently comes mostly from fossil fuels but can be easily obtained in other ways, including electrolysis of water using carbon-free electricity such as solar or wind power.
The researchers tested their system on a real-world example of mixed recycled plastic, yielding promising results. But more testing of more kinds of mixed waste streams is needed to determine how much contamination of different pollutants occurs when materials such as ink, glue, and labels attached to plastic containers or other non-plastic materials are mixed together -- and how it affects the long-term stability of the process.
智能推荐
电化学过程创新思维 | 创新利用“Cryo-EM快照”可改进电池设计
2022-10-17研究人员利用低温电子显微镜拍摄了固体电解液间相(SEI)的第一张高分辨率图像,这为科学家提供了一种潜在的新方法来调整和改进电池设计。
涉及学科涉及领域研究方向储能科学创新思维 | 利用机器学习帮助提高锂离子电池和燃料电池的性能
2022-09-01帝国理工学院的研究人员将机器学习与能源储存相结合,开发出了一种名为“深度卷积生成对抗网络”(DC-GANs)的新技术。研究团队的发现将帮助能源领域的研究人员设计和制造优化电极,以提高电池性能。
涉及学科涉及领域研究方向