创新背景
大气中温室气体的排放和积累导致气候环境不断恶化,加紧处理二氧化碳是保护环境的必需措施,寻找储存方法可以帮助减少二氧化碳的危害。
创新过程
巴塞尔大学生物中心的Ben Engel教授与法兰克福大学Volker Müller教授、马尔堡大学Jan Schuller教授的研究人员合作,研究阐明了一种酶的结构,寻找到一种储存二氧化碳的新方法,相关研究成果《膜锚固的HDCR纳米线驱动氢动力二氧化碳固定》发表在2022年出版的《自然》上。
研究阐明的酶名为HDCR,由长丝组成,呈螺纹状结构,像一个导电的纳米线。HDCR酶最初于1981年在中非的基伍湖发现,存在于嗜热细菌热无菌中,生活在深海等氧气很少的环境中。
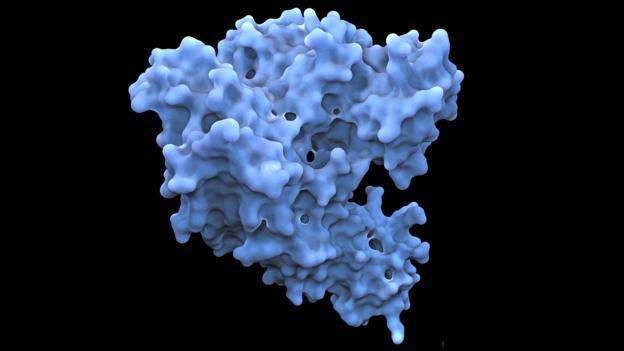
HDCR酶通过将电子从氢气转移到二氧化碳,可以转化气态氢和二氧化碳,达到去除环境中的二氧化碳并将其存储在单元中的目的。HDCR酶的螺纹状结构满足两种气体有效转化的需求。酶的结构域使高速存储二氧化碳成为可能。研究人员表示,HDCR酶比任何先前已知的执行这种反应的化学催化剂都要快。
研究使用冷冻电子显微镜方法探究了解HDCR的工作原理,揭示来自产乙酰菌热无菌kivui的短HDCR细丝的结构。研究先在实验室环境中对纯化的酶的细丝进行成像,从而确定酶的原子结构。细丝的细节清晰呈现出来,展现它如何包含数千个铁和硫原子的电子传导簇,进而使电子能有效地从气态氢传递到二氧化碳。
结构中最小的重复单元是一个由一个甲酸脱氢酶和两个氢化酶组成的六聚体。六聚体围绕着氢化酶Fe-S亚基的中心核心,小细菌聚铁氧化还原素样蛋白通过其C端螺旋寡聚形成细丝的骨架。将结构定向诱变和酶分析结合,研究发现长丝化和通过长丝的快速电子转移增强了HDCR酶的活性。研究人员认为,即使只有一个氢气泡通过细菌时,酶的细丝也能储存来自氢的电子。

成像工作完成后,研究人员使用尖端技术,断层扫描冷冻细胞切片,使T. kivui细胞内的天然HDCR结构成像,发现细丝像金属电缆一样绕着自己旋转了好几次。研究人员认为,细丝自我旋转不仅证实了HDCR细丝在细胞中的发生,而且它们束成附着在质膜上的大环形上部结构。这些结构像是膜上的圆形门户,细菌在这种极端条件下可以获得能量,从而提高酶的工作效率。
HDCR结构展示了一种使用氢气作为原料,有效储存二氧化碳的新方法。
创新价值
研究对未来的生物技术应用极为有用,证明了探索不同生物生物学的基础科学研究的价值。
HDCR enzymes store carbon dioxide through efficient hydrogen conversion
Professor Ben Engel of the University of Basel Biological Center, in collaboration with researchers at the University of Frankfurt Volker Müller and Professor Jan Schuller of the University of Marburg, has elucidated the structure of an enzyme and found a new way to store carbon dioxide, and the research result "Membrane-anchored HDCR nanowires drive hydrogen-powered carbon monoxide 2 fixation" was published in the 2022 issue of Nature.
The enzyme elucidated by the study is called HDCR, which consists of filaments with a thread-like structure, like a conductive nanowire. The HDCR enzyme was first discovered in 1981 in Lake Kivu in Central Africa, where it is found in the thermophilic bacteria, thermotopic bacteria, and lives in environments with little oxygen, such as the deep sea.
By transferring electrons from hydrogen to carbon dioxide, HDCR enzymes can convert gaseous hydrogen and carbon dioxide to remove carbon dioxide from the environment and store it in the cell. The threaded structure of
HDCR enzymes meets the needs of efficient conversion of both gases. The enzyme's domain makes it possible to store carbon dioxide at high speeds. The researchers say the HDCR enzyme is faster than any previously known chemical catalyst to perform this reaction.
The study used cryo-electron microscopy to explore how HDCR works, revealing the structure of short HDCR filaments from acetyl fungus-producing thermo-sterile kivui. The study begins with imaging of the filaments of the purified enzyme in a laboratory setting to determine the atomic structure of the enzyme. The details of the filament are clearly revealed, showing how it contains an electron conduction cluster of thousands of iron and sulfur atoms, which in turn allows electrons to be efficiently transferred from gaseous hydrogen to carbon dioxide.
The smallest repeating unit in the structure is a hexamer consisting of one formate dehydrogenase and two hydrogenases. The hexamer surrounds the central core of the hydrolase Fe-S subunit, and the small bacterial polyferro-redoxadotropin forms the backbone of the filament through its C-terminal spiral oligomerization. Combining structure-oriented mutagenesis and enzyme analysis, the study found that filamentation and rapid electron transfer through filament enhance the activity of HDCR enzymes. The researchers believe that even when only one hydrogen bubble passes through the bacteria, the filaments of the enzyme can store electrons from hydrogen.
After the imaging work was completed, the researchers used cutting-edge technology to scan frozen cell sections to make T. Imaging the natural HDCR structure inside kivui cells, the filaments were found spinning around themselves several times like a metal cable. The researchers believe that filament self-rotation not only confirms the occurrence of HDCR filaments in cells, but also that they are bundled into large ring-shaped superstructures attached to the plasma membrane. These structures are like circular portals on membranes, where bacteria can gain energy under extreme conditions, thereby increasing the work efficiency of enzymes.
The HDCR structure demonstrates a new way to efficiently store carbon dioxide using hydrogen as a feedstock.
智能推荐
化学生物学理论创新 | 生物体都具有甲烷形成的通用机制
2022-08-14通过枯草芽孢杆菌的特性进行对照实验,发现甲烷形成机制的通用性。
涉及学科涉及领域研究方向创新利用CRISPR系统在微重力条件下诊断基因
2022-08-04利用CRISPR系统在微重力环境中实现简单快速诊断病原体,开发太空环境中的疾病治疗方法。
涉及学科涉及领域研究方向融合多种技术手段揭示恐惧消退形成新记忆的动态印迹网络机制
2022-07-29该研究综合运用动物行为学、神经活性捕捉原位标记、光遗传学、神经环路示踪、电生理、在体光纤记录等多种技术手段,针对恐惧消退中形成的新记忆--消退记忆,在记忆印迹细胞群及其互相连接构成的印迹环路和印迹网络层面,揭示了消退记忆从形成到遗忘的基本演化规律,为理解抑郁症、焦虑症、创伤后应激障碍等重大情感障碍疾病治疗后复发提供了全新的思路。
涉及学科涉及领域研究方向利用带有“分子密码”的墨水传递机密文件
2022-08-04研究团队采用256位加密密钥,并将其编码为一种类似塑料的材料——寡聚氨基甲酸酯(oligourethane),得到了一种能够加密大型数据集的新存储介质。这种“分子密码”的出现创造了一种可能性——将密钥隐藏在各式物品的分子结构中,不仅仅是墨水,也可能是纸张、项链、钥匙扣等等。同时,这也是首次将如此多的信息存储在这种类型的聚合物中,它标志着分子数据存储和密码学领域的革命性科学进步。
涉及学科涉及领域研究方向