创新背景
将单个铱原子固定在催化粒子的表面,可以提高催化反应的性能,而这一直是可持续能源生产的瓶颈。
创新过程
这是这种方法第一次被应用于氧析出反应(OER)——电解过程的一部分,利用电力将水分解为氢和氧。如果由可再生能源提供动力,电解可以更可持续地生产燃料和化学原料,并减少化石燃料的使用。但OER的缓慢步伐一直是提高其效率以使其能够在公开市场上竞争的瓶颈。
研究小组表示,这项研究的结果可以缓解瓶颈,并为观察和理解这些单原子催化中心在现实工作条件下如何工作开辟新的途径。
催化剂是化学工业的支柱,为可持续能源的未来带来希望。就像媒人一样,它们从流动的液体或气体中抓住分子,鼓励它们相互反应,而不会被消耗掉。为了最大限度地提高这一过程的效率,催化剂纳米颗粒通常分散在多孔材料的表面,这为许多反应同时发生提供了最大可能的表面积。但只有纳米粒子外部的原子才能参与催化作用;内在的就会被浪费掉。当催化剂是一种昂贵的贵金属,如铱或铂,即使是少量的废物也是昂贵的。因此,科学家们一直在研究使用这些贵金属的单个原子来代替。每个原子都是催化反应中心。它们的微小尺寸意味着在一个给定的支撑结构表面可以容纳更多的它们。这大大增加了暴露在反应物中的活性催化剂的数量,以及可以同时发生的反应的数量,提高了效率。
在这项研究中,由SLAC/斯坦福大学教授Yi Cui和SLAC的工作人员科学家Michal Bajdich领导的团队开发了一种新方法,将单个铱原子锚定在支撑表面上。斯坦福大学博士后研究人员郑雪丽和唐静进行了这项实验,SLAC的副研究员、科学家亚历山德罗·加洛对x射线数据进行了理论模拟,揭示了哪种构型最稳定和有效。
为了制造这种新型催化剂,研究人员首先制作了一个多孔结构来支撑催化反应的铱原子。他们将这种泡沫状结构暴露在含有铱化合物的溶液中,迅速冻结它,在表面形成一层薄的富含铱的冰,并进行额外的处理,以创造出分布均匀的位置,使单个铱原子紧紧地固定在支撑表面上。
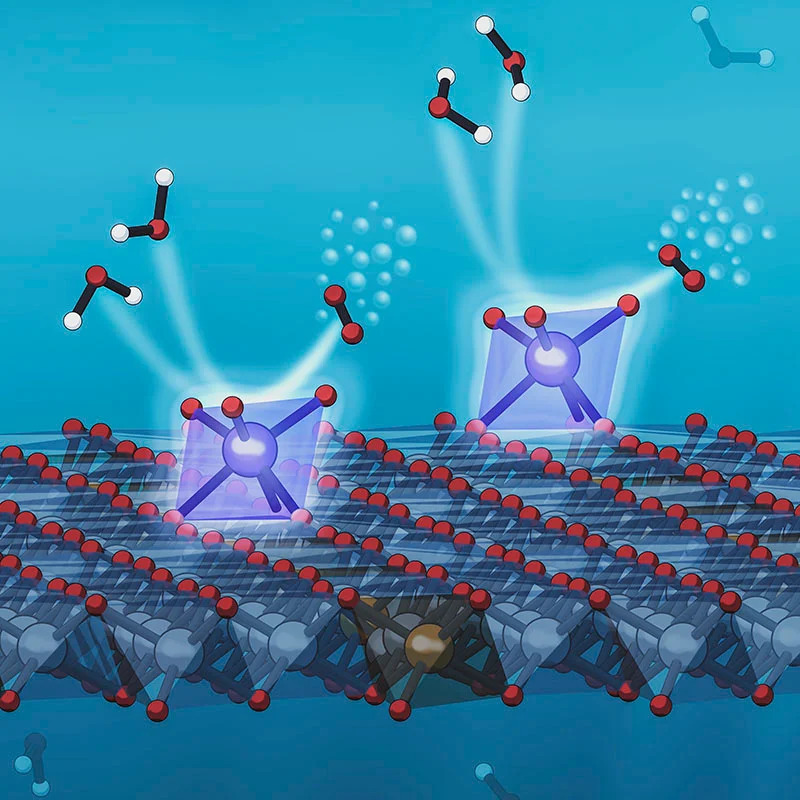
对这种催化剂的x射线观察显示,铱原子处于一种化学状态,这使得它们在水分解反应中释放氧气的部分特别有效。其他的测试表明,这种增强的活性完全是因为铱是以单个孤立原子的形式存在的,而不仅仅是因为它们嵌入的大表面积。
研究人员称,这种催化剂比目前已知的大多数铱基催化剂都要好。他们说,这种新的原子锚定系统为探索和建立催化剂及其支撑结构之间的连接提供了一个理想的模型,用于各种电催化反应。
创新价值
一种新的原子锚定系统为探索和建立催化剂及其支撑结构之间的连接提供了一个理想的模型,用于各种电催化反应。
创新关键点
这是这种方法第一次被应用于氧析出反应(OER)——电解过程的一部分,利用电力将水分解为氢和氧。如果由可再生能源提供动力,电解可以更可持续地生产燃料和化学原料,并减少化石燃料的使用。
创新主体
崔怡是SLAC斯坦福材料与能源科学研究所(SIMES)的研究员,米哈尔·巴季迪奇是SUNCAT界面科学与催化中心的研究员,该中心是SLAC/斯坦福联合研究所,在那里进行了理论计算。对催化剂的x射线观察由SLAC的斯坦福同步辐射光源(SSRL)和劳伦斯伯克利国家实验室的先进光源(ALS)进行,计算工作由国家能源研究科学计算中心(NERSC)进行;这三个都是美国能源部科学办公室的用户设施。来自伯克利实验室分子铸造中心和美国国家标准与技术研究所材料测量设施的研究人员也参与了这项由美国能源部科学办公室资助的工作。
Using new methods to create special monatomic catalysts
This is the first time this method has been applied to the oxygen release reaction (OER), a part of the electrolysis process that uses electricity to split water into hydrogen and oxygen. If powered by renewable energy, electrolysis can produce fuels and chemicals more sustainably and reduce the use of fossil fuels. But OER's slow pace has been a bottleneck to improving its efficiency so that it can compete in the open market.
The team says the results of this study could ease bottlenecks and open new ways to see and understand how these single-atom catalytic centers work under realistic working conditions.
Catalysts are the backbone of the chemical industry and offer hope for a sustainable energy future. Like matchmakers, they grab molecules from a flowing liquid or gas and encourage them to react with each other without being consumed. To maximize the efficiency of this process, catalyst nanoparticles are typically dispersed on the surface of a porous material, which provides the maximum possible surface area for many reactions to occur simultaneously.
But only atoms outside the nanoparticles can be involved in catalysis; What's inside will be wasted. When the catalyst is an expensive precious metal, such as iridium or platinum, even small amounts of waste are expensive. So scientists have been working on using individual atoms of these precious metals instead. Each atom is a catalytic reaction center. Their tiny size means that more of them can fit on the surface of a given supporting structure. This greatly increases the number of active catalysts exposed to the reactants, as well as the number of reactions that can occur simultaneously, improving efficiency.
In this study, a team led by SLAC/ Stanford professor Yi Cui and SLAC staff scientist Michal Bajdich developed a new method to anchor a single iridium atom to a supporting surface. Xueli Zheng and Jing Tang, postdoctoral researchers at Stanford University, conducted the experiment, and scientist Alessandro Gallo, an associate researcher at SLAC, performed theoretical simulations on X-ray data that revealed which configuration was most stable and efficient.
To make the new catalyst, the researchers first made a porous structure to support the iridium atoms that catalyze the reaction. They exposed the foamlike structure to a solution containing iridium compounds, quickly froze it, forming a thin layer of iridium-rich ice on the surface, and subjected it to additional processing to create evenly distributed positions that would hold individual iridium atoms tightly to the supporting surface.
X-ray observations of the catalyst show that the iridium atoms are in a chemical state that makes them particularly efficient at releasing the oxygen part of the water decomposition reaction. Other tests have shown that the enhanced activity is due entirely to the iridium being in the form of single isolated atoms, and not just to the large surface area in which they are embedded.
The researchers say the catalyst is better than most iridium-based catalysts known so far. They say this new atomic anchoring system provides an ideal model for exploring and establishing connections between catalysts and their supporting structures for a variety of electrocatalytic reactions.
智能推荐
新材料 | 使用钒和阳光的化学反应分解塑料
2022-06-29利用钒金属和光能分解塑料,并转化降解物促进电能利用。
涉及学科涉及领域研究方向化学合成非织造布抗菌纺织品
2022-06-29创新使用射频气体放电等离子体技术开发新技术,改善非织造布材料性能,增加其抗菌作用。
涉及学科涉及领域研究方向新能源 | 利用铁、碳和氢创新融合帮助氢燃料电池降低成本
2022-06-29利用已有廉价易得元素进行分离式单原子金属转化合成生产新型化学催化剂。
涉及学科涉及领域研究方向从沸石反应中发现可将残余甲烷转化为甲醇的方式
2022-08-14从沸石自然反应中发现“笼效应”,仿生研究甲烷羟基化的机制。
涉及学科涉及领域研究方向