创新背景
虽然研究人员对酶已经有了很多了解,包括它们的结构和它们用来促进反应的化学基团,但关于它们的形式如何与它们的功能相联系的细节,以及它们如何以如此惊人的速度和特异性实现它们的生化魔法,仍然没有得到很好的理解。
创新过程
福迪斯和她在斯坦福大学的同事们开发了一项新技术,并在本周的《科学》杂志上详细介绍了这项技术,这项技术可能会帮助改变这种状况。这项技术被称为HT-MEK,是高通量微流体酶动力学的缩写,它可以通过同时进行数千次酶实验,将多年的工作压缩到几周内。

HT-MEK可以让科学家深入探索酶中发生底物结合的小“活性位点”之外,揭示酶中最遥远的部分如何协同工作以实现其显著的反应性的线索。
HT-MEK的设计是为了取代传统的费力的酶纯化过程,传统的纯化过程涉及改造细菌产生一种特定的酶,在大烧杯中培养它们,打开微生物,然后从所有其他不需要的细胞成分中分离出感兴趣的酶。为了拼凑出一种酶的工作原理,科学家们故意在它的DNA蓝图中引入错误,然后分析这些突变如何影响催化作用。
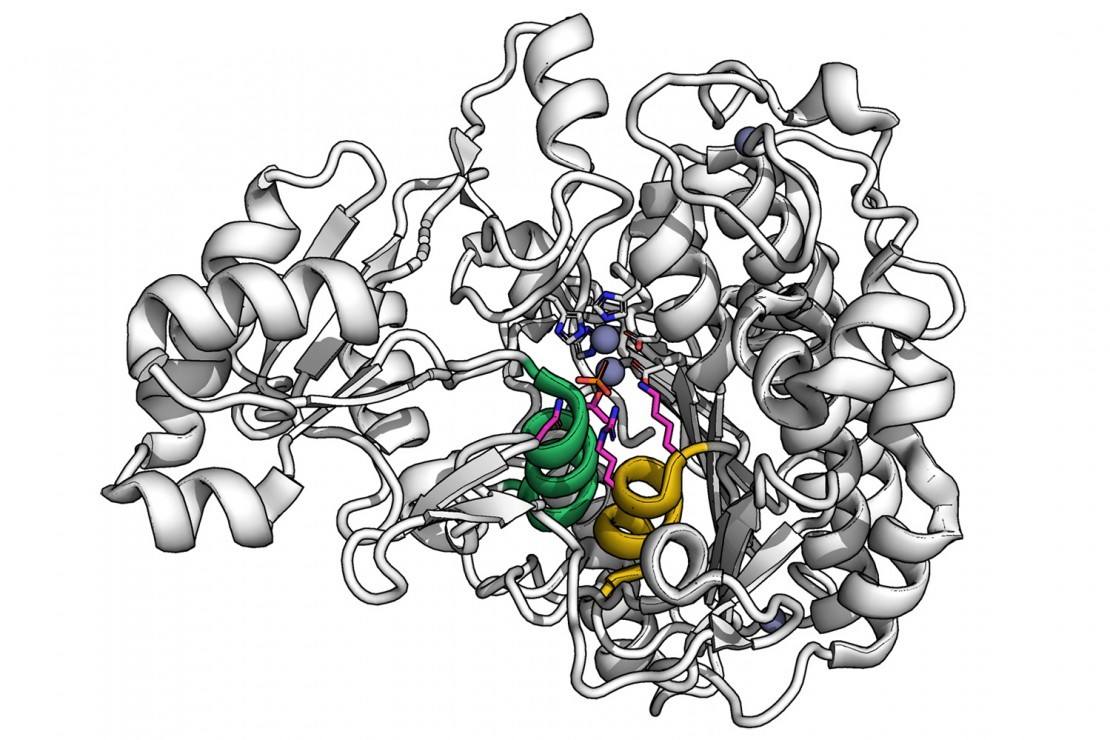
HT-MEK使科学家能够轻松地将他们的目光转移到酶活性位点以外的部分,并探索,例如,改变酶表面的形状可能如何影响其内部的工作。
HT-MEK结合了两种现有的技术来快速加快酶分析。第一种是微流体,它涉及到模压聚合物芯片来创建用于精确操作流体的微观通道。微流体缩小了进行这些流体实验的物理空间,就像集成电路减少了计算所需的空间一样。第二种是无细胞蛋白质合成技术,这种技术只需要那些生产蛋白质所需的生物机械的关键部件,并将它们组合成一种可以用于合成酶的糊状提取物,而不需要活细胞充当孵育器。
由于每一个微小的腔室只包含千万分之一升的物质,科学家们可以在一个设备中设计数千种酶的变体,并并行研究它们。通过调整每个腔室的DNA指令,他们可以修改组成这种酶的氨基酸分子链。通过这种方式,我们有可能系统地研究一种酶的不同修饰是如何影响其折叠、催化能力以及结合小分子和其他蛋白质的能力的。
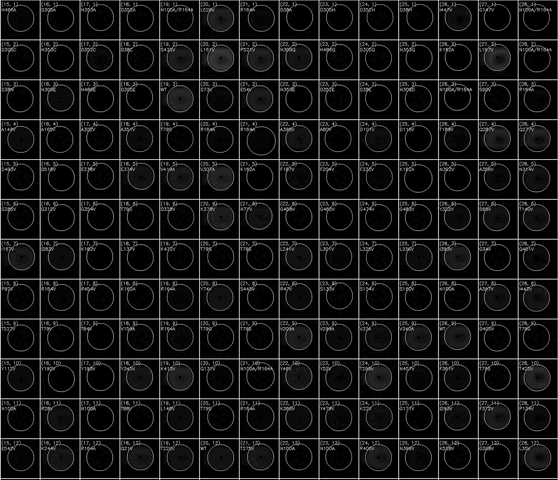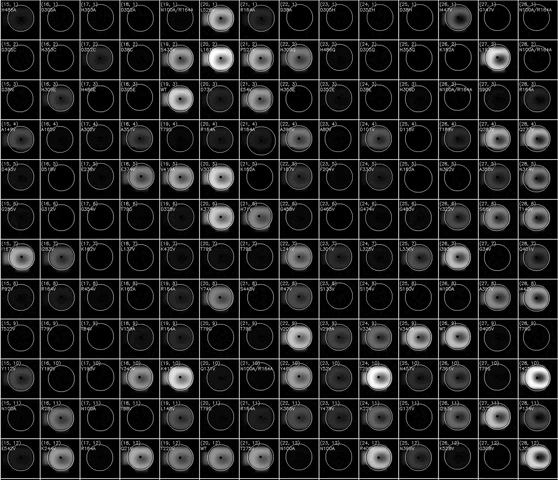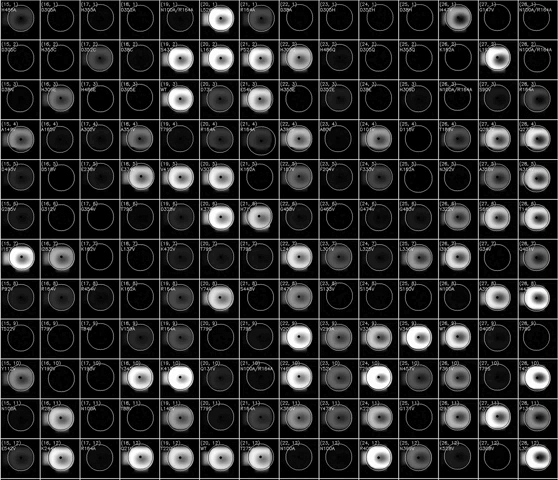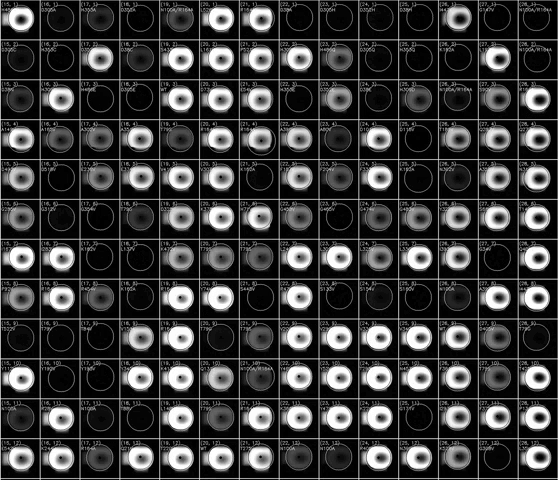
(动图显示荧光积聚表示HT-MEK器件的一部分随时间推移的催化反应)
当该团队将他们的技术应用到一种被称为paa的酶上时,他们发现活性位点以外的突变会影响其催化化学反应的能力——事实上,组成这种酶的大部分氨基酸或“残基”都有影响。
科学家们还发现,数量惊人的突变导致paa错误折叠到一种不能进行催化的状态。该研究的共同第一作者克雷格·马尔金(Craig Markin)说:“生物化学家几十年前就知道错误折叠可能会发生,但要确定这些案例极其困难,定量估计这种错误折叠物质的数量就更难了。”马尔金是福代斯和赫施莱格实验室的联合研究员。
创新价值
如果被广泛采用,HT-MEK不仅可以提高研究人员对酶功能的基本认识,还可以催化医药和工业的进步。HT-MEK还可以加速一种称为变构靶向的药物开发方法,HT-MEK产生的海量数据也将有利于计算方法和机器学习算法。
创新关键点
HT-MEK结合了两种现有的技术来快速加快酶分析。第一种是微流体,它涉及到模压聚合物芯片来创建用于精确操作流体的微观通道。第二种是无细胞蛋白质合成技术。
Innovation and development of new tools to accelerate the speed of enzyme research
A new technique developed by Fordis and her colleagues at Stanford and detailed in this week's issue of the journal Science could help change that. The technique, known as HT-MEK, short for High-throughput microfluidic enzyme kinetics, can compress years of work into a few weeks by performing thousands of enzyme experiments simultaneously.
Ht-mek allows scientists to probe beyond the small "active site" in the enzyme where substrate binding occurs, revealing clues about how the most distant parts of the enzyme work together to achieve its remarkable reactivity.
Ht-mek is designed to replace the traditional laborious enzyme purification process, which involves engineering bacteria to produce a specific enzyme, growing them in large beakers, opening up the microorganisms, and then isolating the enzyme of interest from all other unwanted cellular components. To piece together how an enzyme works, scientists deliberately introduce errors into its DNA blueprint and then analyze how those mutations affect catalysis.
Ht-mek allows scientists to easily shift their gaze to parts of the enzyme beyond its active site and explore, for example, how changing the shape of the enzyme's surface might affect its inner workings.
Ht-mek combines two existing technologies to rapidly accelerate enzyme assays. The first is microfluidics, which involves molding polymer chips to create microscopic channels for precise fluid manipulation. Microfluidics shrink the physical space in which to conduct these fluid experiments, just as integrated circuits reduce the space required for computation. The second is cell-free protein synthesis, which takes only those key components of biomechanics needed to produce proteins and combines them into a paste extract that can be used for synthetases, without the need for living cells to act as incubators.
Since each tiny chamber contains only 10 millionths of a liter of material, scientists can design thousands of enzyme variants in a single device and study them in parallel. By tweaking the DNA instructions for each chamber, they can modify the chain of amino acid molecules that make up the enzyme. In this way, it is possible to systematically investigate how different modifications of an enzyme affect its folding, catalytic capacity, and ability to bind small molecules and other proteins.
When the team applied their technique to an enzyme called PAA, they found that mutations outside the active site affected its ability to catalyze chemical reactions - in fact, most of the amino acids, or "residues," that make up the enzyme.
The scientists also found that a surprising number of mutations caused PAA to misfold into a state where it could not be catalyzed. "Biochemists have known for decades that misfolding can occur, but it is extremely difficult to identify these cases and even harder to quantitatively estimate the amount of such misfolded material," said Craig Markin, co-first author of the study. Malkin is a co-investigator at the Fordyce and Hirschlaeger LABS.
智能推荐
从沸石反应中发现可将残余甲烷转化为甲醇的方式
2022-08-14从沸石自然反应中发现“笼效应”,仿生研究甲烷羟基化的机制。
涉及学科涉及领域研究方向利用微小气泡来帮助治疗常见的儿童癌症
2022-08-01伦敦大学学院(UCL)的研究人员已经开发出一种新方法,可以提供药物,关闭神经母细胞瘤中促进癌症的突变。
涉及学科涉及领域研究方向利用细菌可将二氧化碳转化为化学品
2022-08-04利用细菌菌株开发气体发酵工艺,回收二氧化碳制作丙酮和异丙醇。
涉及学科涉及领域研究方向新型光激活涂层用于杀死细菌
2022-08-04伦敦大学学院(ucl)领导的研究团队开发出了一种可以在低强度的光线下激活,杀死金黄色葡萄球菌和大肠杆菌等细菌的新型涂层。
涉及学科涉及领域研究方向