创新背景
病毒是大自然的特洛伊木马:它们进入细胞,偷运遗传物质,利用细胞自身的机制进行复制。几十年来,科学家们一直在研究如何最大限度地减少它们的有害影响,甚至重新利用这些入侵者传递的不是它们自己的病毒基因组,而是治疗疾病的疗法和研究细胞的工具。然而,要想在这些新角色中发挥作用,每一种病毒必须安全、有效、有选择地作用于那些需要其基因载体的细胞。这是一项病毒的天然储备无法胜任的任务。
创新过程
加州理工学院的研究人员开发了一种方法,可以快速有效地识别设计腺相关病毒(AAV)变体,这种病毒可以传递到或“转导”小鼠特定类型的细胞,使科学家能够根据研究或临床需要选择一种病毒。天然AAV是这项工作的一个特别有希望的起点,因为尽管该病毒在人群中广泛流行,但没有已知的疾病关联,不会引起强烈的免疫反应,也不能自行繁殖。
所有的病毒都有相同的基本设计:它们是由一种叫做衣壳的外壳保护的遗传物质片段,要么是RNA,要么是DNA。AAV衣壳的表面化学成分会影响其附着细胞膜蛋白的能力,从而使其能够进入细胞。在天然病毒中,衣壳已经进化到能够吸附在存在于许多不同组织或细胞群表面的蛋白质上。然而,为了使它们只对需要治疗的细胞有效,生物工程师们致力于设计衣壳,使其能够进入特定的细胞类型。这是可能的,如果设计衣壳可以锁住膜蛋白,只有在一种细胞类型中发现,而不是其他细胞类型。

脑脉管系统在这里以红色成像,通过将荧光基因传递给特定细胞而成为可能。
为此,研究人员一直致力于为不同的细胞类型和用途组装病毒载体目录,方法是利用定向进化的力量来调查单个宿主中数百万个AAV衣壳变体。在新的研究中,他们开发了一种叫做M-CREATE(基于多路复合Cre重组的AAV靶向进化)的方法。这种方法快速筛选大量AAV衣壳库,每个衣壳都有一个独特的表面环装饰,以获得与不同细胞蛋白的相互作用,从而进入小鼠的特定细胞类型。
这项工作建立在Gradinaru实验室开发的一种早期方法的基础上。2016年,该团队确定了如何设计AAV通过血脑屏障(BBB)的方法。血脑屏障是一层紧密包裹的细胞层,通常可以阻止循环病毒和其他病原体进入大脑和脊髓。改良后的病毒载体现在可以通过简单的全身静脉注射进行注射,使研究人员可以避免侵入性的大脑注射,并已被广泛采用为研究工具。然而,之前的筛选过程耗时费力,只识别了少数衣壳,无法进行跨多个靶点的筛选,从而发现多个组织的衣壳特异性,进而发现这些组织内的不同细胞类型。
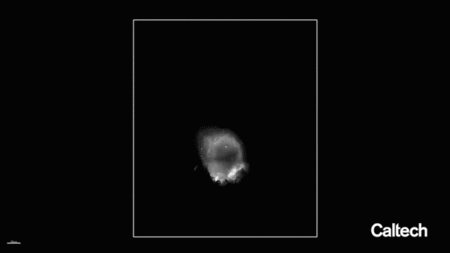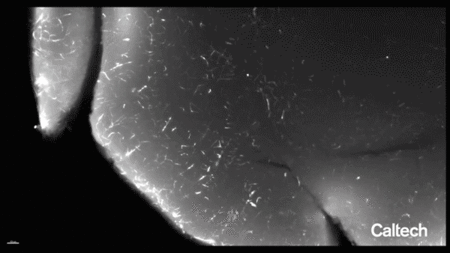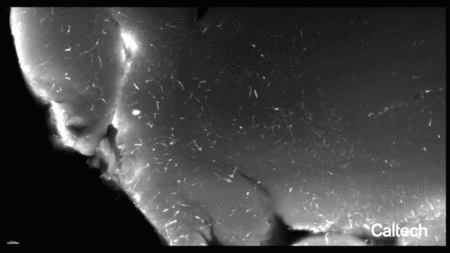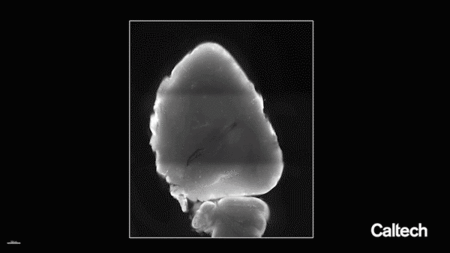
一种含有荧光分子基因的工程病毒衣壳被输送到成年小鼠的血液中,该小鼠经过基因改造以在其内皮细胞中表达一种名为Cre的蛋白质。荧光基因的表达依赖于Cre的表达,因此只有内皮细胞才会发出荧光。然后清除一个脑半球并进行标记,以便在大组织体积中可视化完整的脉管系统。
专门针对与疾病相关的神经元和非神经元脑细胞(如组成血管系统和血脑屏障的脑内皮细胞)的基因传递工具,可以使神经退行性变或中风等领域的研究范式发生转变。由于血脑屏障受损会导致病理因素进入大脑,引发和(或)加速神经退行性变,通过工程AAVs对血脑屏障通透性进行功能靶向,可以通过循环影响身体到大脑的通路。这将使人们能够修复疾病中被削弱的屏障,或者相反,如果有必要,可以暂时渗透健康的血脑屏障,以便通过血液向大脑提供治疗。
研究人员还发现了许多AAV衣壳,它们可以以类似的效率穿过血脑屏障,尽管它们在衣壳中有高度不同的序列。与之前工程的AAV显示出一些菌株特异性不同,新的衣壳在具有不同血脑屏障特征的小鼠的测试菌株中同样有效。这表明,新的工程衣壳可以通过与独特的表面蛋白质相互作用通过血脑屏障。识别获取同一组织的不同作用机制可能对这些工具从小鼠转化为包括人类在内的其他模型生物至关重要。
创新关键点
M-CREATE的创新点在于它有潜力识别具有独特表面结构的不同衣壳,这些衣壳可以解决相同的问题,比如以不同的方式穿过血脑屏障。
创新价值
新技术M-CREATE仅通过衣壳就可以对AAV进行细胞类型特异性的工程改造,不需要基因调控元件来实现特异性,因此可以在小的AAV衣壳中为所需的遗传物质释放关键空间。
Innovative development "M-CREATE method" can quickly identify AAV variants
Caltech researchers have developed a method to quickly and efficiently identify designer adeno-associated virus (AAV) variants that can be delivered to, or "transduced" to, specific cell types in mice, allowing scientists to select a virus for research or clinical needs. Natural AAV is a particularly promising starting point for this work because although the virus is widespread in the human population, it has no known disease association, does not elicit a strong immune response, and cannot reproduce on its own.
All viruses have the same basic design: they are fragments of genetic material, either RNA or DNA, protected by a shell called a capsid. The surface chemistry of the AAV capsid affects its ability to attach to cell membrane proteins, allowing it to enter the cell. In natural viruses, capsids have evolved to attach to proteins present on the surface of many different tissues or cell populations. However, in order to make them effective only on cells that need treatment, bioengineers are working on designing capsids that can enter specific cell types. This is possible if designed capsids can lock in membrane proteins found only in one cell type and not others.
To this end, researchers have been working to assemble catalogues of viral vectors for different cell types and uses by harnessing the power of directed evolution to investigate millions of AAV capsid variants in a single host. In the new study, they developed an approach called M-CREATE (Targeted Evolution of AAV Based on multiplex Cre Recombination). This approach rapidly screens a large library of AAV capsids, each with a unique surface ring decoration, for interactions with different cellular proteins that enter specific cell types in mice.
This work builds on an earlier method developed at Gradinaru's lab. In 2016, the team determined how to design AAV to cross the blood-brain barrier (BBB). The blood-brain barrier is a tightly packed layer of cells that normally prevents circulating viruses and other pathogens from entering the brain and spinal cord. Modified viral vectors can now be injected via simple systemic intravenous injections, allowing researchers to avoid invasive brain injections, and have been widely adopted as research tools. However, previous screening processes were laborious and identified only a few capsids, making it impossible to screen across multiple targets to discover the capsid specificity of multiple tissues and, consequently, the different cell types within those tissues.
Gene delivery tools that specifically target both neuronal and non-neuronal brain cells associated with disease, such as brain endothelial cells that make up the vasculature and blood-brain barrier, could lead to a paradigm shift in research in areas such as neurodegeneration or stroke. Because BBB damage can cause pathological factors to enter the brain and trigger and/or accelerate neurodegeneration, functional targeting of BBB permeability through engineered AAVs can affect the body to brain pathway through circulation. This would allow people to repair weakened barriers in disease or, conversely, to temporarily penetrate the healthy blood-brain barrier if necessary in order to deliver treatments to the brain via the blood.
The researchers also found a number of AAV capsids that could cross the BBB with similar efficiency, despite having highly different sequences in the capsids. Unlike previously engineered AAVs that showed some strain specificity, the new capsid was equally effective in test strains from mice with different BBB features. This suggests that new engineered capsids can cross the BBB by interacting with unique surface proteins. Identifying different mechanisms of action for acquiring the same tissue may be crucial for the translation of these tools from mice to other model organisms, including humans.
智能推荐
生物医学新材料 | 创新利用“冷冻丝绸”帮助修复受损心脏
2022-09-29生物医学工程师在完善了一种制造能促进心脏干细胞生长的生物材料的方法后,朝着为受损心脏开发心脏补丁的目标又迈进了一步。
涉及学科涉及领域研究方向生物医学工程创新 | 利用活细胞构建“半互穿聚合物网络”使药物输送更安全
2022-09-30杜克大学的生物医学工程师已经证明了一种被称为半互穿聚合物网络的交织复合材料可以由活细胞产生。
涉及学科涉及领域研究方向生物医学工程创新 | 创新利用铝制成可在液态金属中分解的医学设备
2022-11-09麻省理工学院的研究人员利用一种导致金属断裂的现象,设计了一种更简单的移除不再需要的医疗设备的方法。
涉及学科涉及领域研究方向生物医学工程创新 | 将抗生素涂层创新应用于骨科植入物可降低感染机会
2022-09-28杜克大学的生物医学工程师等人发明了一种抗生素涂层,可以在手术前几分钟涂在骨科植入物上,从而消除植入物周围感染的机会。
涉及学科涉及领域研究方向